SharePoint and Power Platform for Quality Management System (eQMS)
Your Microsoft Environment Fully Equipped for eQMS
In the medical device industry and other highly regulated sectors, Quality Management System (QMS) digitalization is no longer optional; it’s essential. Organizations need tools that are flexible, secure, and compliant, while also being user-friendly and scalable.
Microsoft SharePoint and Power Platform (Power Apps, Power Automate, and Power BI) offer a powerful combination that allows businesses to build modern, digital QMS solutions tailored to their processes. With these technologies, you can streamline compliance, automate documentation, and create an intuitive environment for your quality teams. And that all in the environment that you already know!

SharePoint: Trusted Infrastructure for Your Quality Management System
Designed primarily for document storage and management, SharePoint meets the highest enterprise standards, offering seamless collaboration, version control, and access management. Choosing SharePoint means trusting a proven, scalable platform that supports your organization’s operational resilience and digital transformation goals.
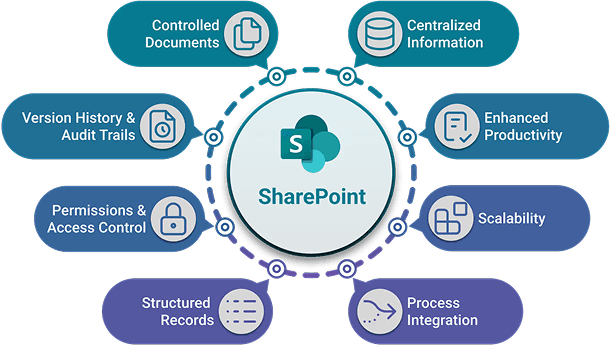
Centralized Document Management
SharePoint enables organizations to leverage an infrastructure they already know and trust. There is no need for a disruptive migration, teams can build workflows and automate processes step-by-step on top of their existing data. Used by thousands of businesses globally for storing and managing all types of documents, SharePoint is the perfect base for your eQMS.
Collaboration with Security
SharePoint provides an enterprise-grade level of security, access control, and reliability. Advanced permission management, real-time collaboration, and seamless integration with Microsoft apps allow teams to work on QMS content securely, with complete visibility on changes and communication.
Long-Term Reliability
With Microsoft’s ongoing support, backup strategies, and proven business continuity and disaster recovery measures, SharePoint offers unmatched reliability. Choosing SharePoint means partnering with a company whose infrastructure and commitment will remain dependable for years to come.
Ensuring Regulatory Compliance with SharePoint as Your eQMS
While Microsoft SharePoint is not a “turnkey” regulated system out-of-the-box, it provides a secure, flexible, and compliant foundation for building a digital Quality Management System (eQMS) that meets regulatory requirements. With the right configuration, policies, and governance, SharePoint can support compliance with standards and regulations critical for the medical device, pharmaceutical, and other highly regulated industries.
Data Security & Access Control
SharePoint leverages Microsoft 365 enterprise-grade security, including encryption at rest and in transit, multi-factor authentication, and role-based access control.
Audit Trails & Version Control
SharePoint tracks document changes, approvals, and user activity, enabling traceable audit trails critical for regulatory inspections
Data Retention & Records Management
SharePoint’s information management policies allow organizations to define retention schedules, record types, and lifecycle rules, supporting regulatory documentation requirements.
Integration with Compliance Tools
SharePoint works seamlessly with Microsoft Purview, Teams, and Power Platform to ensure comprehensive data governance and compliance monitoring.

Quality Management System for Medical Devices

Electronic Signatures & Audit Trails

In Vitro Diagnostic Regulation

Medical Device Regulation

Health Insurance Portability and Accountability Act

General Data Protection Regulation

Good Practices
We combine SharePoint with the Microsoft Power Platform and robust governance to deliver a fully compliant digital QMS environment; helping organizations meet regulatory standards, maintain continuous audit readiness, and ensure complete traceability across all quality processes.

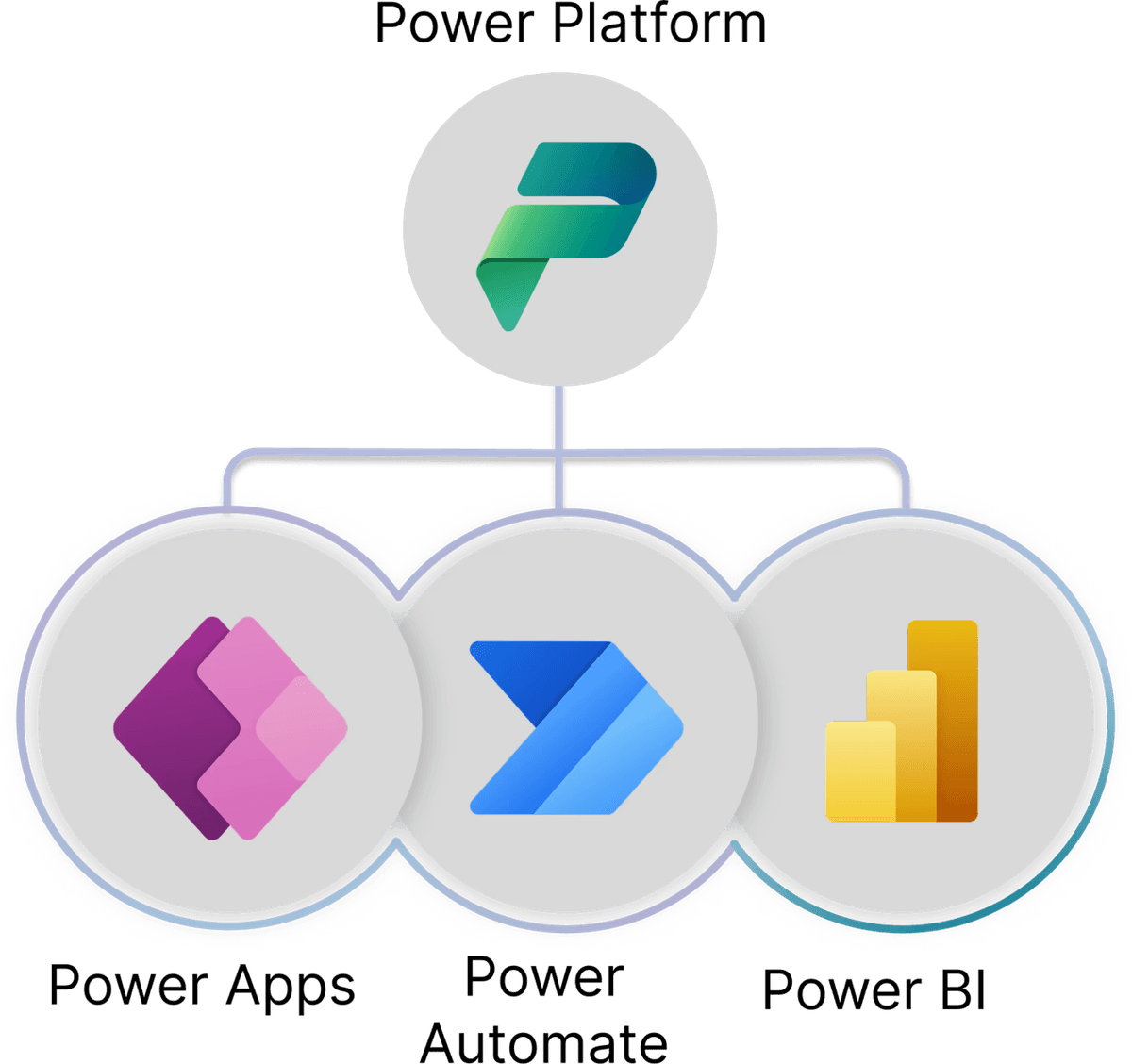
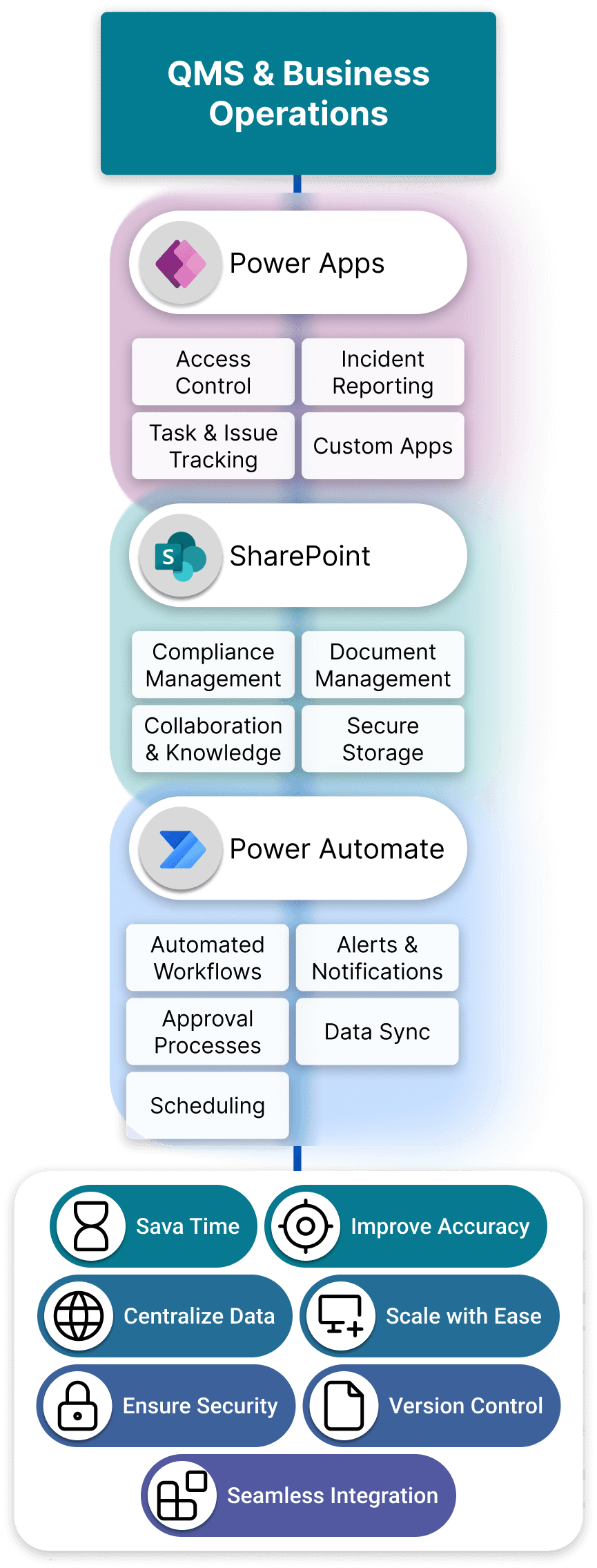
Microsoft Power Platform Enhance & Customize Your eQMS
Microsoft Power Platform is a suite of applications that enhances SharePoint-based QMS by enabling automation, data-driven insights, and custom app development. It is particularly effective in regulated industries like medical devices, where efficiency, going hand-in-hand with compliance, and traceability is critical.
At QMLogic, we design and build custom Power Apps solutions that transform complex QMS processes into simple, compliant, and user-friendly digital workflows, built on top of SharePoint.
Our expertise lies in creating regulated environments that extend SharePoint with intuitive interfaces, built-in validation, and full traceability.
We develop applications that fit your organization’s structure and approval logic, ensuring seamless integration with Microsoft 365 and your QMS.
A Connected Digital QMS Ecosystem
Together, Power Apps, Power Automate, and Power BI form a connected, compliant digital quality ecosystem.
QMLogic delivers the expertise to design, implement, and validate every layer, ensuring your organization achieves automation, audit readiness, and regulatory compliance without disrupting existing workflows.

Power Apps Solutions:Tailor eQMS to Fit Your Business
Microsoft Power Apps is a low-code/no-code platform that allows organizations to build custom applications quickly and efficiently. In the context of Quality Management Systems, Power Apps provides the flexibility to digitize processes that standard SharePoint features or off-the-shelf QMS software cannot fully address.
Instead of forcing your quality processes into rigid pre-built systems, Power Apps lets you design apps that mirror your workflows — whether it’s managing audits, training records, CAPAs, or customer complaints. These apps connect directly to SharePoint and Microsoft 365, ensuring secure data storage, version control, and compliance with regulatory standards.
Key Benefits of Power Apps for eQMS
User-Friendly Interfaces
Build intuitive apps with modern UIs that simplify adoption across quality and compliance teams.
Custom Workflows for Compliance
Support ISO 13485, FDA 21 CFR Part 11, EU MDR, and other regulations with workflows that reflect your exact processes.
Adaptable & Future-Proof
Apps evolve with your business and regulatory requirements without the limitations of rigid off-the-shelf systems.
Strategic Benefits of Custom Power Apps in eQMS
Rapid Development & Deployment
Power Apps allow to deliver functional QMS applications much faster than traditional custom-coded development.
Full Data Ownership & Security
All data stays within your Microsoft 365 environment, secured by Microsoft’s enterprise-grade standards.
No Vendor Lock-In
If you ever move away from the solution, your apps and data remain yours, ensuring long-term flexibility and independence.

Power Automate: Compliance-Driven Digitalization Customized to You
Power Automate connects every part of your Microsoft ecosystem QMS, from SharePoint and Power Apps to Teams, Outlook and OneDrive, creating a seamless flow of information across departments.
We design automation that fits your processes, not the other way around. Tasks can trigger automatically from document updates, CAPA changes, or audit findings, while scheduled workflows monitor deadlines and maintain oversight across your QMS.
With hundreds of Microsoft 365 and third-party connectors (Teams, SAP, JIRA, supplier portals), we integrate your tools into a unified, compliant system that eliminates manual work without disrupting your established tools or workflows.
What our automation delivers:
Event and schedule-based triggers for actions and reminders
Fully traceable, audit-ready process execution
Streamlined document and task management across teams
Real-time monitoring of deadlines and CAPA status
Reduced manual effort and human error
Typical automations in QMS and operations:
Document Control
Route SOPs or work instructions for review and approval with digital signatures.
CAPA Escalation
Send automated reminders or escalation notices when CAPA deadlines are approaching or missed.
Supplier Qualification
Automatically collect supplier responses, store documentation in SharePoint, and notify quality managers when action is required.
Audit Preparation Workflows
Generate audit checklists, notify stakeholders, and compile evidence automatically.

Power BI:Turning QMS Data Into Intelligence
We transform your quality data into powerful visual insights that drive smarter decisions. With Power BI, we build validated, audit-ready dashboards that bring clarity to every aspect of your QMS, from KPIs and management reviews to nonconformities, CAPAs, and supplier performance.
Our Power BI experts design connected reports that analyze trends, track performance, and highlight recurring issues before they become risks. Whether you’re preparing for an audit, evaluating process effectiveness, or monitoring continuous improvement, you’ll always have the data you need, accurate, real-time, and accessible.
Examples in QMS and Operations
CAPA Effectiveness Dashboard
Track CAPA closures, overdue items, and long-term effectiveness metrics.
Training Compliance Reports
Visualize training completion rates, upcoming expirations, and competency gaps across teams.
Supplier Performance Dashboard
Monitor supplier quality, on-time delivery, and audit results.
Regulatory Audit Readiness Dashboard
Compile key compliance indicators to demonstrate readiness for FDA, ISO, or notified body inspections.

For Larger Organizations
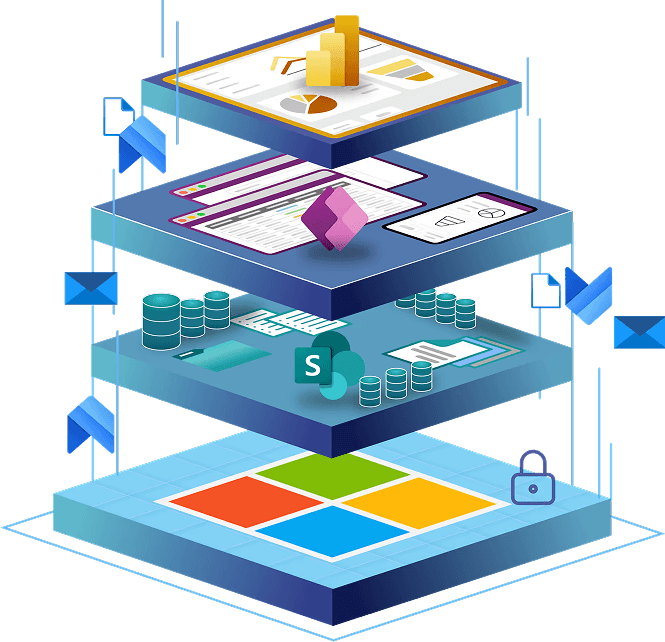
How SharePoint & Power Platform Work Together to Improve Your QMS
When integrated, SharePoint and Power Platform form a connected digital ecosystem for Quality Management. Instead of relying on rigid, off-the-shelf software, you can build a QMS that mirrors your processes, ensures compliance, and adapts as your business evolves.
The real strength of Microsoft’s ecosystem is not in the individual tools, but in how they interconnect to form a single digital quality environment. Each platform plays a different role, and together they eliminate gaps between data, processes, and people.
The Layers of the Microsoft Quality Ecosystem
SharePoint as the Backbone

SharePoint serves as the secure repository and collaboration hub, it centralizes documents, lists, and records while enforcing permissions, version control, and compliance rules.
Power Apps as the User Experience Layer

Instead of relying on SharePoint’s native forms, Power Apps provides flexible, mobile-ready interfaces. This makes complex quality processes intuitive for end users while still writing data directly back to SharePoint.
Power Automate as the Connective Tissue

Power Automate keeps processes in motion. Actions in SharePoint or Power Apps can trigger workflows that route approvals, send notifications, synchronize data, or connect with external systems.
Power BI as the Intelligence Layer

Once processes are running and data is flowing; Power BI turns it into actionable insights. Reports and dashboards can be embedded directly in SharePoint, providing managers with oversight and compliance monitoring in real time.
Practical QMS Interactions Across Platforms
SharePoint + Power Automate


An item is added to a SharePoint list (e.g., a new deviation record), and Power Automate automatically starts a workflow, routing it for approval, sending reminders, and logging every step for audit purposes.
SharePoint + Power Apps


Power Apps provides user-friendly forms and interfaces on top of SharePoint lists and libraries. For example, employees can log incidents via a mobile app, with all data securely stored in SharePoint for version control and compliance.
SharePoint + Power BI


Power BI dashboards can be embedded directly into SharePoint pages, allowing managers to track CAPA status, training compliance, or supplier performance in real time, all without leaving their QMS portal.
Full Integration Across the Platform




A change request entered in Power Apps is stored in SharePoint, routed through Power Automate for approvals, and visualized in Power BI dashboards. Every component works together, delivering a seamless, traceable QMS process.
This connected approach ensures that every quality process — from document control to audits and CAPAs — is digital, automated, and data-driven, all built on the security and compliance foundation of Microsoft 365.
Examples of QMS Software Solutions with SharePoint and Power Apps
Every QMS process can be digitized. From document control to audits, training, or complex workflows like our Computer System Validation (CSV) solution, SharePoint and Power Platform can transform manual tasks into streamlined digital solutions.
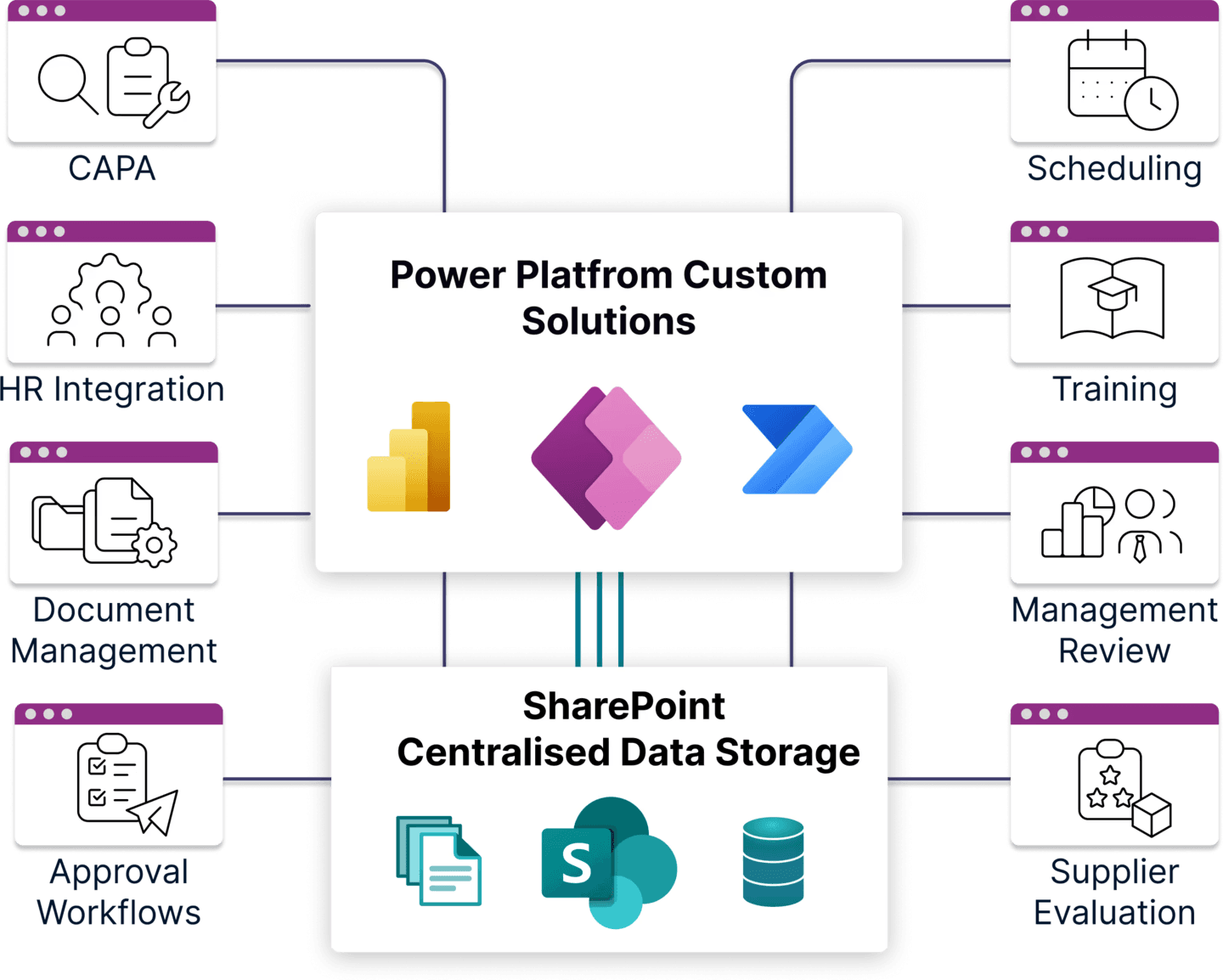
Advantages of Using SharePoint & Power Platform for Quality Management
Full Control by Your Organization
With SharePoint and Power Platform, you retain full control over your applications and data. Data stays within your Microsoft 365 environment; no vendor lock-in.
Seamless Integration
If your company already uses Microsoft products, the integration is seamless and cost-effective. These tools work with your existing Microsoft ecosystem, including Teams, Outlook, Excel, and other Microsoft 365 tools.
Cost-Effective Implementation
Unlike specialized QMS software with high recurring license fees, SharePoint and Power Platform rely on your existing Microsoft 365 subscription. In most cases, the tools are already available in your organization, making digital QMS adoption faster and more budget-friendly.
Enterprise-Grade Security
Powered by Microsoft’s robust security infrastructure, all your data is protected by enterprise-grade security. Authentication is managed through Microsoft, so your team can log in using the same credentials they use for Outlook and other Microsoft services.
Minimal Disruption
Incremental adoption ensures a smooth transition without interrupting critical QMS activities. There’s no need for a big bang solution; customize at your own pace, and scale as you grow.
Modular & Scalable
Build small, tailored solutions for each area of your QMS. Whether it’s project management, HR, or sales, you can customize the platform to meet your specific needs and expand as your QMS matures.
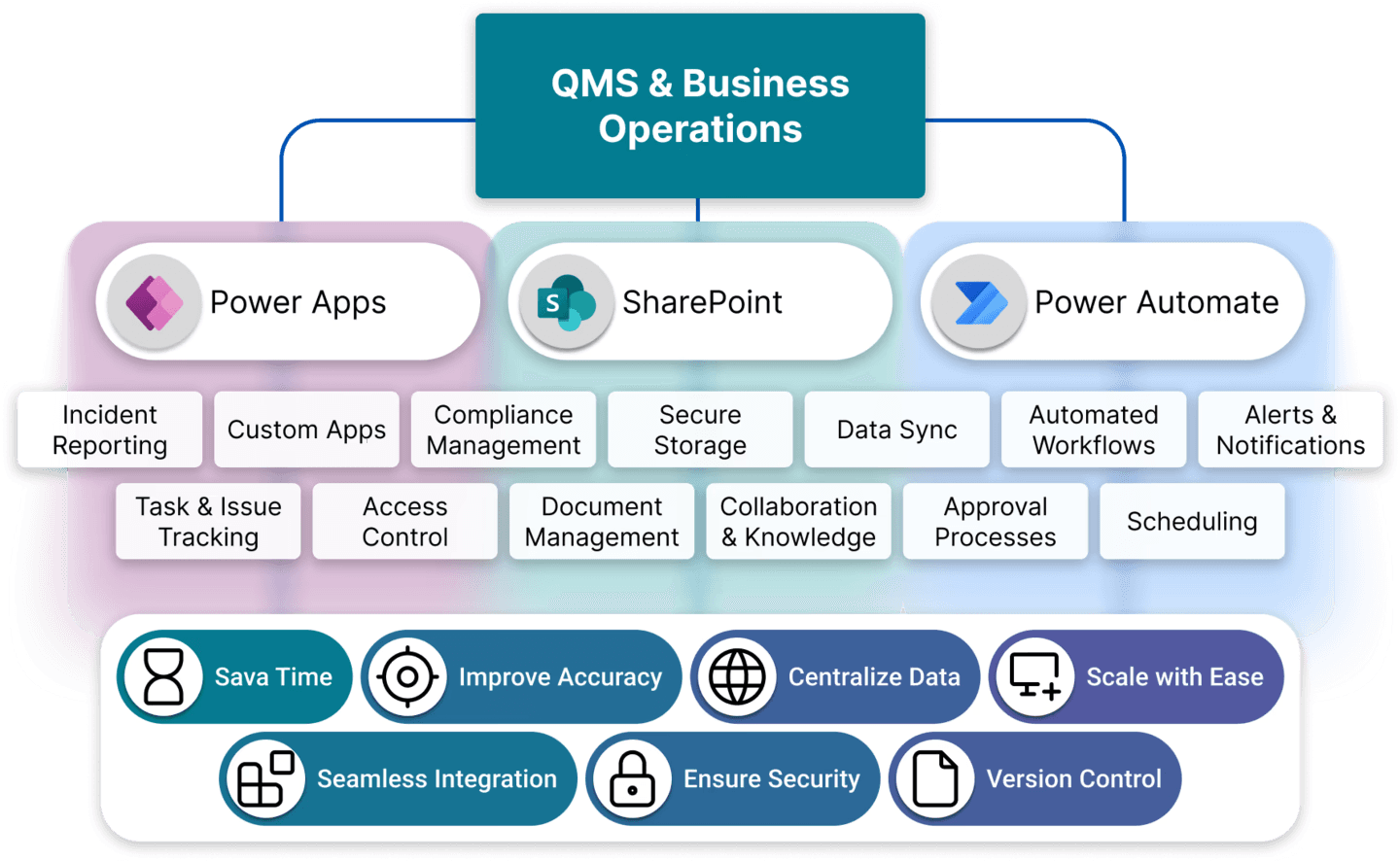
Extending Beyond SharePoint & Power Platform
While Power Apps, Power Automate, and Power BI cover the majority of use cases, some advanced scenarios may require deeper integration or custom development.
This ensures your digital QMS ecosystem is not only flexible but also future-ready—capable of handling complex requirements.

Using External Connectors & APIs
Integrating with third-party systems like ERP, CRM, or MES.

Leveraging the SharePoint Framework (SPFx)
Creating custom components and user experiences within SharePoint.

Utilizing Microsoft Graph API
Enabling secure, scalable integrations across Microsoft 365 services.
Advanced Integrations for SharePoint & Power Platform
We also extend SharePoint and Power Platform with APIs, SPFx, and Graph API to deliver advanced, compliant QMS solutions tailored to your needs.




Testimonials | Expertise in QMS Digitalization
Vaclav did support our journey to become ISO 13485 certified with relentless work put into great processes, great communication and detailed and to the point conversations with the teams, individual members - but also our external auditors. He was of great support to move our QMS to the next level. He was the key person behind the move away from paper / scan based documents towards a digital setup.If you are in need for a structured person with great communication skills, a good understanding of the regulatory environment, I can only recommend to reach out to Vaclav. Lucky you if he's not booked ;-) Besides all the success he's an enjoyable and humble character. Great experience to having worked with him.
Vaclav and I worked together on risk management activities of medical device development projects. His ability to quickly grasp the challenges and to accomplish a task/project is remarkable. Additionally, his ability to propose and develop software solutions to improve projects’ efficiency is commendable. I find Vaclav to be flexible, dependable, and quality centric. Hence, I really enjoyed working with Vaclav and looking forward to associate with him again.
Questions & Answers
Yes, we can help you choose the right solution for you, either because you just decided to leave your current vendor, or you are first establishing a quality management system.
Together, we will analyze your current situation and define the requirements that are most important to you, and we can advise you which quality management system solution to choose.
We have the most experience in building quality management systems around the Atlassian or Microsoft suite, but we can evaluate any other software solution that would fit your needs.
For specific requirements and organizational setups, we also provide custom-made software solutions, fully developed by us.
Our Other Services
Regulatory Consulting for SaMD/MDSW
Ensure complete MDR, FDA, IEC 62304, ISO 14971, and cybersecurity compliance.
We understand the regulatory environment. We have experience in implementing all standards related to medical software development, cybersecurity, and technical documentation, and we would be happy to demonstrate in practice how we can translate this expertise into real-world solutions while working on your medical device software product - as a developers a project managers.
- Real-World Solution for Your Organization
Medical Device Software Development (SaMD/MDSW)
Medical Device Software Development
QMLogic delivers trusted medical device regulatory consulting services and medical device regulatory compliance software support, guiding you from development to successful certification.
- Software Development
- Technical Documentation
- Medical Project Management
- Regulatory Compliance
Get consultancy for free
Ask anything you need to know about Medical Software, CE certification or MDR.

