Medical Device Software Development (SaMD/MDSW)
End-to-End Medical Device Software Development
| From Concept to Certification
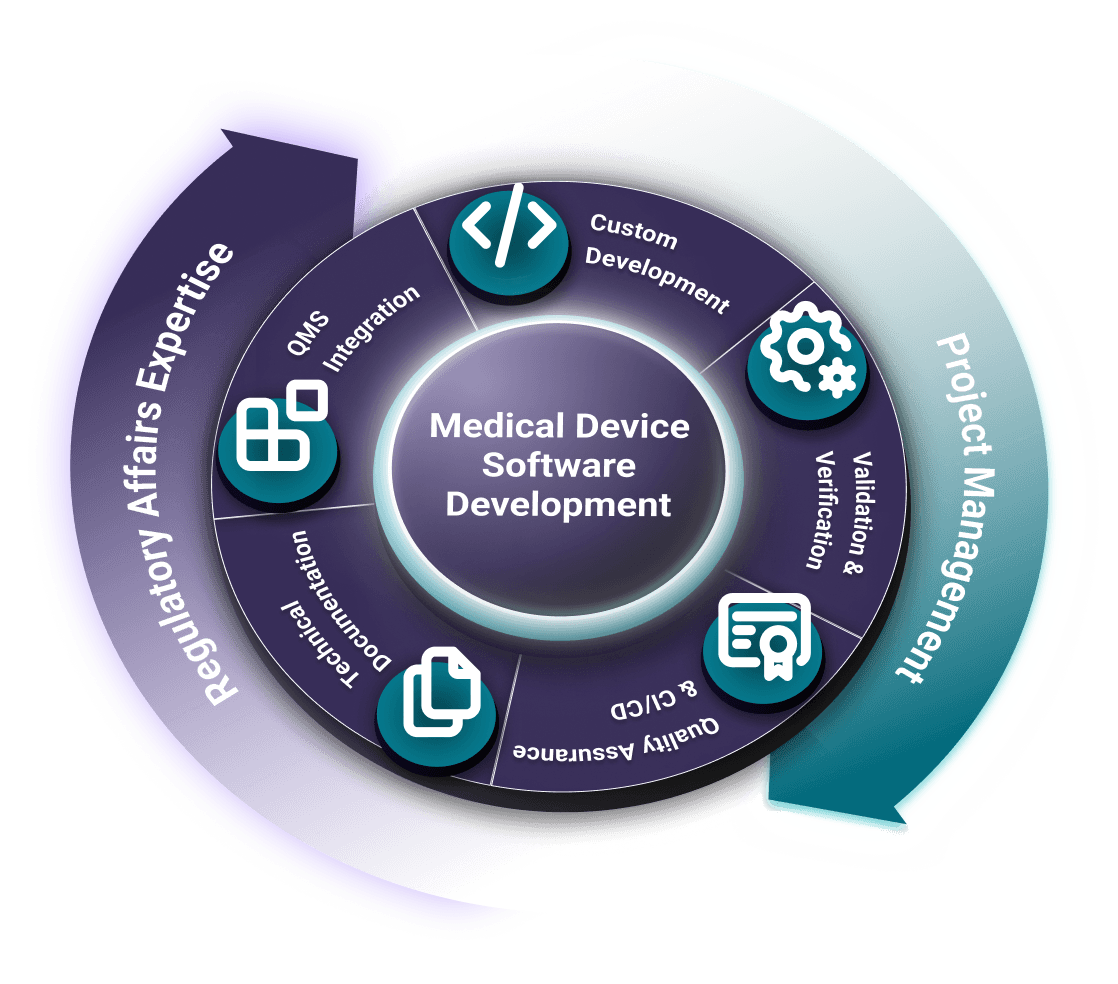
QMLogic is your trusted partner for Medical Device Software (MDSW/SaMD) development. We provide complete technical, regulatory, and project management support, ensuring your medical device software is safe, effective, and compliant with international standards and regulations such as IEC 62304, ISO 13485, ISO 14971, FDA 21 CFR, and EU MDR.
From concept and design, verification and validation to regulatory submission, and post-market surveillance, we guide medical device companies through the entire software development lifecycle. Our solutions are always audit-ready, compliant, and seamlessly integrated into your Quality Management System (QMS).

Medical Device Software Development Services
We are developers by DNA. Our team builds daily sophisticated software using cutting-edge technologies like React.js, Next.js, Spring Boot, and .NET. Development is not something we outsource; it's our core expertise, seamlessly integrated with our regulatory knowledge to create compliant, innovative solutions from day one.
- Compliance built into every line of code
- Developers and regulatory experts in one team
- IEC 62304 compliant by design
- Integrated with ISO 14971 Risk Management
- Designed with Cybersecurity per IEC 81001-5-1
- React, Spring Boot, .NET mastery
- Modern stack, medical-grade security
We build software aligned with your quality management system, ensuring every line of code supports compliance, efficiency, and business continuity.
Regulatory Expertise in Medical Device Development
Our regulatory affairs expertise ensures that your software is audit-ready, validated, and compliant with key standards, including:

IEC 62304
Software Lifecycle Compliance
Defines the required development and maintenance processes for SaMD/MDSW, ensuring safety, traceability, and regulatory approval.

ISO 13485
QMS for Medical Software
Provides the quality management framework to align software development with global medical device standards.

ISO 14971
Risk Management Integration
Ensures risk identification, evaluation, and control are embedded into the software lifecycle, protecting patients and users.

ISO 80002-2
Computer Systems Validation
Guides computer system and software validation, ensuring reliable performance and regulatory acceptance.

EU-MDR
European medical device regulation
Sets the requirements for CE marking of medical device software, covering safety, performance, and post-market surveillance.

21 CFR Part 820
U.S. Compliance
Part 11 governs electronic records and signatures, while Part 820 ensures quality system compliance for FDA-regulated software.

FDA 510(k)
Market Access Clearance
Demonstrates substantial equivalence of your medical device software, enabling U.S. market entry.

ISO/IEC 27001
Information Security
Provides a globally recognized framework for managing information security, ensuring medical device software protects sensitive health data.

IEC 81001-5-1
Health Software Cybersecurity
Specifically addresses cybersecurity for health software, focusing on secure design, development, and maintenance of medical software.
By uniting technical excellence with regulatory mastery, we help MedTech companies:
Design, build, and validate SaMD with regulatory compliance built in
Develop audit-ready technical documentation from the start
Streamline pathways to EU MDR certification and FDA clearance
Launch faster with a scalable and compliant software solution
Our unique approach ensures that every line of code supports not just your business goals, but also the regulatory framework that governs your market success. From concept to certification, we help you design, build, validate, and document your SaMD for a faster, smoother market launch.
Why QMLogic for Medical Device Software Development?

Proven Track Record of Success
We have delivered multiple successful and fully compliant SaMD development projects, from initial concept to post-market surveillance, for startups and established MedTech companies alike.
End-to-End Project Management Integration
Our dedicated project management services ensure efficient coordination, transparent communication, milestone tracking, and proactive risk management across the entire development lifecycle.
Regulatory Expertise Across Global Markets
Extensive knowledge of IEC 62304, ISO 13485, ISO 14971, EU MDR, and FDA 21 CFR 820 ensures your software meets all necessary regulatory and compliance requirements worldwide.
Cybersecurity and Risk Management
Every phase of development is tightly aligned with ISO 14971 risk management and IEC 81001-5-1 cybersecurity standards, ensuring patient safety and data protection from the ground up.
Customizable and Scalable Solutions
Whether you are launching an MVP, scaling to a complex platform, or entering new global markets, our development and compliance approach can flexibly scale to meet your evolving business and technical needs.
QMS Integration & Digitalization
Your software development processes will seamlessly align with your ISO 13485-compliant Quality Management System (QMS), and we also offer support for QMS software implementation and digitalization to modernize your operations.
Ready to Develop Your Medical Device Software?
Whether you are a startup launching your first SaMD or an established manufacturer expanding your portfolio, we help you accelerate development while ensuring compliance.
Testimonials | Expertise in Medical Device Software
Vaclav and I worked together on risk management activities of medical device development projects. His ability to quickly grasp the challenges and to accomplish a task/project is remarkable. Additionally, his ability to propose and develop software solutions to improve projects’ efficiency is commendable. I find Vaclav to be flexible, dependable, and quality centric. Hence, I really enjoyed working with Vaclav and looking forward to associate with him again.
I had the pleasure to work with Vaclav during our development of a medical product for Merck. I found Vaclav to be very professional and devoted. His attention to detail and his technical /medical knowledge brought immense valuable inputs to the project. Collaborate with Vaclav was really a pleasure and I warmly recommend him.
Your Trusted Partner in All Steps of Medical Device Software Lifecycle
Medical Device Regulation
Efficacy and feasibility is as important as compliance
We understand the regulatory environment. We have experience in implementing all standards related to medical software development, cybersecurity, and technical documentation, and we would be happy to demonstrate in practice how we can translate this expertise into real-world solutions while working on your medical device software product - as a developers a project managers.
- Real-World Solution for Your Organization
Quality Management Digitalization
and Automation for ISO 13485
At QMLogic, we empower organizations to revolutionize their Quality Management Systems (QMS) with cutting-edge automation tools. Our expertise spans Microsoft SharePoint, Power Platform, Confluence, Jira, and custom-built solutions tailored to specific needs.
- Future of Quality Management
Get consultancy for free
Ask anything you need to know about Medical Software, CE certification or MDR.

