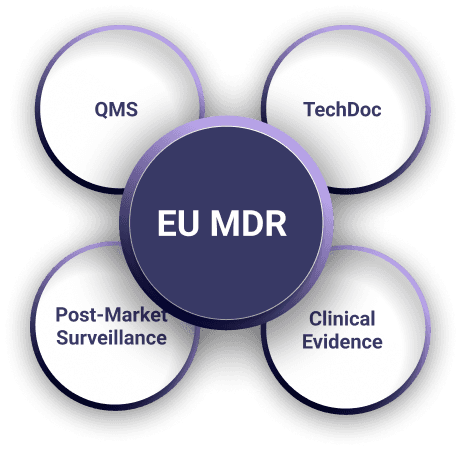
EU MDR Expertise for SaMD & Digital Health
We specialize in EU MDR consulting for software and connected devices, including:
- Standalone SaMD (Software as a Medical Device)
- Embedded medical software and integrated platforms
- Cloud-based and AI-driven digital health applications