
ISO 13485
Medical Device Compliance

Medical Device Compliance

Guiding the Software Lifecycle

Managing Risks Effectively

Addressing Cybersecurity Risks

Medical Device Regulation

Compliance & Cybersecurity

Organizational Data Security

Health AI Systems

Aligning with U.S. Standards

AI Management Systems

U.S. Quality System Regulation

In Vitro Diagnostic Regulation

Computer System Validation
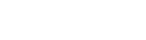
Corrective and Preventive Actions

Quality System Evaluation

We help you implement a quality and regulatory environment for your medical device software that is not just compliant but also works for your organization. Such a solution will reflect your philosophy, structure, and daily reality, connecting people, processes, and objectives into one functioning unit.
We recognize that compliance is not the only priority in your organization. You have your business plans, release deadlines, and team objectives. We don’t place regulatory or quality activities above all others; instead, we demonstrate how every department can collaborate to achieve its goals in alignment: be fast, compliant, and efficient at the same time.
We will guide you through specific standards, but our real focus is on helping you to see how all those standards connect and work together as one coherent system.
We understand that your goal is not only to be compliant but also to succeed in the market, staying innovative, effective, and fast enough to outpace the competition while delivering safe, high-quality products.
Mutual understanding between people and departments is essential. We believe that every team in your company cares about quality; sometimes, they just see it differently in terms of what quality means or how it can be achieved. We help align those perspectives so that quality becomes a shared language, not a point of friction.
We will help you create a compliant environment that your employees understand, where they work faster, communicate better, and enjoy being part of a meaningful and modern quality management system.
QMLogic offers a complete complete consulting services for the medical device software lifecycle.
The standards we work with form the foundation of compliance for any company developing software in a regulated medical device environment.
Over the years, we have supported our clients in many areas, from building quality management systems and shaping regulatory strategies to implementing risk management and cybersecurity processes and integrating them into modern eQMSsolutions.
Through this work, we have built deep expertise across the complex ecosystem of standards and regulations for both the European and American markets.
This allows us to offer our clients comprehensive consulting for medical device software, combining both regulatory and technical perspectives.

Each of the main medical device standards and regulations addresses a specific aspect of the same reality. They are not isolated documents; together they define a connected network of activities that cover the entire software lifecycle, from design and development, through maintenance, to monitoring effectiveness and safety in the market.
Instead of letting processes and standards exist side by side, we integrate them together, ensuring that your organization works as one well-orchestrated system.
We implement standards into a practical framework tailored to your organization's needs.

We combine regulatory knowledge with a practical reflection on how your organization develops, including its size, structure, and pace of growth.
Every company we work with is at a different stage of its journey. Some are building their first systems, others are scaling fast after investment, and many are optimizing mature quality environments.
We adapt to your context, your size, structure, and priorities, to design solutions that make sense for you now and create a foundation for where you want to go next.
We approach compliance with practicality. For smaller teams, it means helping you establish what’s essential and effective without unnecessary complexity. For larger organizations, it’s about increasing efficiency, avoiding redundant activities, and keeping compliance fully integrated into complex company operations.
Regulatory compliance will be built around your organization’s dynamics.
Our focus is always on connection: teams, systems, and the alignment of business and regulatory objectives. The result is a quality environment that evolves naturally with your organization.
We don’t stop at explaining what needs to be done; we help you make it happen
We act as an extension of your team, translating regulatory requirements into practical actions.
Our team will help you:
From regulatory concept to working process; making compliance tangible.
Our involvement covers the entire medical device software lifecycle, from the first idea to post-market activities:
Defining product classification and compliance pathway.
Ensuring technical documentation and records are aligned with standards.
Building robust files and integrating them into your product lifecycle.
Supporting continuous compliance and performance monitoring.
Whether you need to design, connect, or transform specific parts of your regulatory and compliance environment, we help you do it efficiently and in line with all relevant regulations, such as ISO 13485, ISO 14971, IEC 62304, MDR 2017/745, and 21 CFR 820.
Implementing and maintaining compliance in the medical device software industry is no longer possible without a modern, digital foundation.
Interpretation of standards, consulting, and even implementation efforts lose their long-term value if they are not supported by an effective electronic Quality Management System (eQMS).
Many companies hesitate to digitalize their QMS, often fearing that tools won’t fit their structure or won’t pass regulatory audits or FDA inspections.
But true risk lies not in transformation; it lies in stagnation. The absence of a properly implemented eQMS leads to regulatory overhead, inefficiency, and growing maintenance costs.
We help you:
We believe that long-term compliance can only be achieved through functional and monitored digital solutions.
These modern QMS tools ensure that your organization remains compliant while staying agile, modern, and efficient.
Your QMS defines how your organization operates. But writing a process is only the first step. The real transformation happens when these processes come to life within a modern digital environment that is used by your people every day.
Let’s build together a modern, connected infrastructure for your organization; one that supports all standards seamlessly and turns your QMS into a system of operational excellence, not just compliance.
Transformation happens when processes stop being described and start being lived.
We provide complete medical device software consulting and compliance implementation services that cover the full journey, from defining your regulatory strategy and designing compliant internal processes to implementing all relevant standards and regulations within one coherent, interconnected system.
The set of standards and regulations is closely related; they address similar challenges from different perspectives, and when implemented together, they naturally reinforce one another.
In the following sections, we illustrate how these frameworks connect, how compliance with one strengthens your alignment with others, and why we focus specifically on this set of standards.
The following standards are the ones most relevant to medical device software, and are the ones every organization will encounter when building a compliant, safe, and modern medical device organization.
Compliant, consistent, and efficient environment that avoids redundancy and unnecessary complexity.
At QMLogic, we empower organizations to revolutionize their Quality Management Systems (QMS) with cutting-edge digitalization and automation tools. Leveraging Jira, Confluence, SharePoint, Power Platform, and custom-built solutions, we design efficient, automated, and ISO 13485-compliant systems tailored to your unique needs.
Medical Device Software Development
QMLogic delivers trusted medical device regulatory consulting services and medical device regulatory compliance software support, guiding you from development to successful certification.

Information
Contact Details